PTD Validation and Clinical Studies
The PTD speaks your bodies Language
The PTD provides direct communication "with the body’s health system"
“Our health relies on the continuous exchange of information within the body. Each and every body function relates to the next in harmony until stress, injury or disease disrupts that communication"
If the process information is correct then health is supported. If the process information is incorrect there may a disturbance to health and signs may show.
Contents
Introduction
PTD Basis
The basis for Medical Certification
CE Legal Indications for Use
The development, beta and final proving tests
The pre clinical trials
The ongoing clinical trials targeting formal medical condition approval.
Sample Test Results
Case Example of EIS 5 Day Test:
Case Example of EIS 30 Minute Test:
Recent Case study example using the ESTech
Supportive Test Methods
Introduction
The PTD has been in successful operation as part of the beat testing program for 2 years with approx. 400 devices sold. Much of this has been in Scandinavia where the PTD is sold via 140 Quantum Medicine clients for ongoing treatment. There are often reviews of the progress via the EIS device (a CE medically certified device for therapeutic follow up. The ongoing results are not yet collected or correlated.
There are three sources of validation information:
- The development, beta and final proving tests.
- The pre clinical trials
- The ongoing clinical trials targeting formal medical condition approval.
- Thousands of clients measured with EIS and several other technologies before PTD and after 2 weeks use.
- Main devices used were the EIS/EStech and Introspect/Oberon to measure specific effects.
- Devices measuring affiliated elements (Meridian, Heart rate Variability, Aura etc.) have also been used.
- More than 100 clinics have tested on their clients for 2-3 years, using symptomatic evaluation as well as medical diagnostic tools or other technologies.
- Results from 5 preliminary trials at hospitals have lead to the initiation of 5 big and publishable studies.
- Current research projects also include: further extensive in house testing, inclusion in a stem cell research project in USA, a Norwegian Government funded study.
PTD Basis
The basis of the PTD is informational: in supporting normal wellness processes whose failure can manifest specifically. Essentially the device works with optimising wellness processes in the specific selected area. It is classified as stress related device as this is the most appropriate of available categories for regulatory processes. It also matches as the PTD is (on the informational/software level) correcting a damaged stress response process, offering patterns to restore a correct and healthy response.
The basis for Medical Certification
Medical certification has a specific set of requirements to be fulfilled. This is not a requirement for informational system which do not act on a direct physiological, biochemical or chemical basis. However from a marketing perspective the acceptance by regulatory authorities that it can provide support for specific medical conditions is beneficial for marketing.
CE Legal Indications for Use
The PTD is a personal therapy device which shares electromagnetic information with your body for the purpose of promoting the self-healing functions.”
AND
This is exactly what it does!
The development, beta and final proving tests
The EIS was mainly used for this and for tuning of the mechanism. This testing was multi centre and whilst results were centrally reviewed only a few typical have been consolidated into reports.
The pre clinical trials
At the request of a specific country several PTD units were provided in 2009. A study was supervised by Medically Qualified Professors using Medical Doctors. The results were positive but a formal report is still awaited. We are not able to specify the parties involved due to confidentiality constraints.
The ongoing clinical trials targeting formal medical condition approval
The above country has signed a contract with the manufacturer for ongoing clinical trials progressively for all programs. This is a full study consistent with all the processes and procedures for world wide acceptability including approved assessment, ethics review and informed consent. The initial focus areas are:
- Elder Adults (Geriatrics)
- Oncology
- Stomatology
- SOMA (Osteomyoarticular System)
- Venous Insufficiency
The effectiveness of the PTD can be demonstrated using an EIS device. The EIS (Electro Interstitial Scan) is approved in USA, Europe and several other countries as a medical device for monitoring the effects of any treatment.
Main Assessment Devices
The EIS (Electro Interstitial Scan) and ESTeck are CE Medical Class 2A devices classified for therapeutic follow up. They essentially show organ functionality (hyper/over functional, hyp/under functional as well as interstitial pH- key minerals- principal hormones, neurotransmitters. This enabled the initial validation of frequencies and along with client feedback forms the main ongoing assessment approach.
The Introspect/Oberon was used to assess specific programs including detoxification (via organ stress activity and reduction in certain toxins), enhancement of parasites reduction, reductions in organ stress levels, scores for inflammatory processes.
Sample Test Results
A sample of some of the many tests done showing the effect of the PTD can be seen below:
Case Example of EIS 5 Day Test
Test Person: Male 61 year old. Feels ok, but white blood cell count is low, and there are some minor complaints about digestive system and respiratory system. First EIS test results are in the left column together with the date of the test. Here the EIS shows a percentage below normal of the state of the tested organ or system. Some variation in organ activity is normal during the day, and also from day to day, but major (greater than 20%) negative percentage shifts show a problem. A reduction in the negative percentage shows an improvement in that organ or system.
In this case the EIS indicates reduced cellular activity in the whole digestive system, as well as the respiratory system. The date and time of the measurements are next to the percentage modulations, so only 5 days have passed in-between these measurements. The test itself is 96% reproducible. The only change the client made in these 5 days was to include daily use of 3 PTD programs:
1)“Regulation of digestive organs” 2) “Regulation of Lungs / Bronchi” 3) “Regulation of Adrenal Gland”.
Digestive system
Program used: “Regulation of digestive organs”, once a day for 5 days
| Functional Status ( - is below normal) | ||
| 17.08.09 | 22.08.09 | |
| Esophagus | -33% | -11% |
| Stomach | -33% | -11% |
| Duodenum | -33% | -11% |
| Small intestine | -45% | -18% |
| Ceacum | -34% | -14% |
| Ascending colon | -34% | -14% |
| Transversal colon by liver | -37% | -19% |
| Transversal colon by spleen | -37% | -14% |
| Descending colon | -45% | -21% |
| Sigmoid colon | -45% | -21% |
| Liver, right lobe | -30% | -21% |
| Liver, left lobe | -38% | -21% |
| Pancreas | -29% | -7% |
Respiratory system
Program used: “Lungs / Bronchi”, once a day for 5 days
| 17.08.09 | 22.08.09 | |
| Trachea and upper bronchi | -27% | -11% |
| Upper lobe of right bronchi/lung | -35% | -14% |
| Middle lobe of right bronchi/lung | -37% | -17% |
| Lower lobe of right bronchi/lung | -39% | -20% |
| Upper lobe of left bronchi/lung | -23% | -04% |
| Lower lobe of left bronchi/lung | -36% | -19% |
Adrenals
Program used: “Adrenal Gland”, once a day for 5 days.
| 17.08.09 | 22.08.09 | |
| Right adrenocortical | -37% | -20% |
| Right medullo-adrenal | -40% | -21% |
| Left adrenocortical | -37% | -20% |
| Left medullo-adrenal | -40% | -21% |
The above test shows significant improvements in the readings for the tested organs and systems due to the use of the PTD over a five day period. The EIS also has a graphic output which indicates clearly the location and degree of improvement indicated by the numbers listed above. It is also possible to demonstrate that the PTD can have an effect in the short term as well.
Case Example of EIS 30 Minute Test
Patient 48 years old, several unresolved emotional factors have caused reduced activity in the hypothalamus, which results in psychosomatic regulatory problems. The PTD program “Hypothalamus / Pituitary” was used once with time reduced to half of the default. An EIS test was performed right before and right after running the program. An imbalance of cellular activity at the level of 24% reduced to 13% in less than half an hour. This is however a temporary balancing, which will only hold for hours or days unless the causation is also dealt with.
Recent Case study example using the ESTech
 |
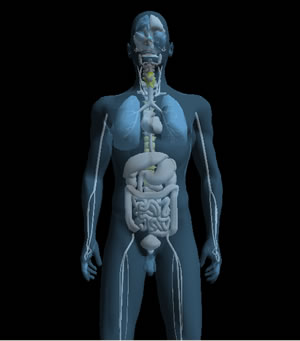 |
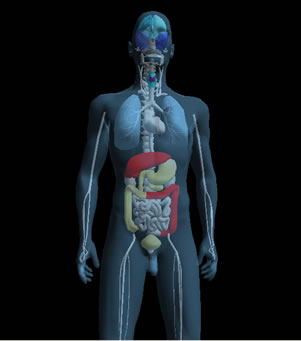
| ES-Teck test performed on Male, 34 in May. All 9 measured areas are out of balance. | ES-Teck test performed on Male, 34 in June, after using PTD according to the test in May. |
ES-Teck is a medically approved device for monitoring effects of medications and therapy.
ES-Teck test performed on Male, 34 in May. All 9 measured areas are out of balance
ES-Teck test performed on Male, 34 in June, after using PTD according to the test in May.
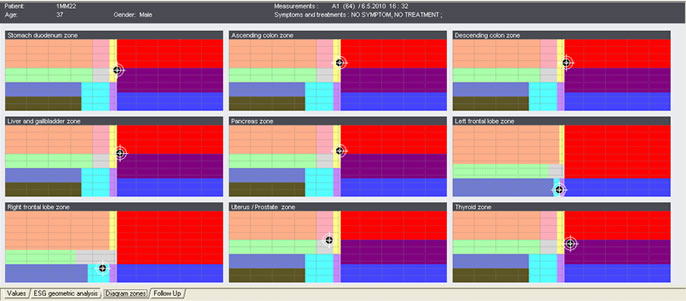
ES-Teck test performed on Male, 34 in May. All 9 measured areas are out of balance.
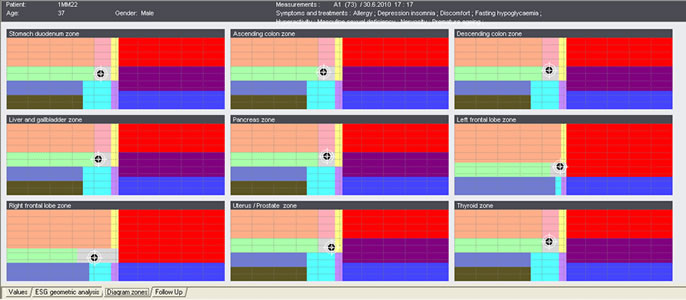
ES-Teck test performed on Male, 34 in June, after using PTD according to the test in May.
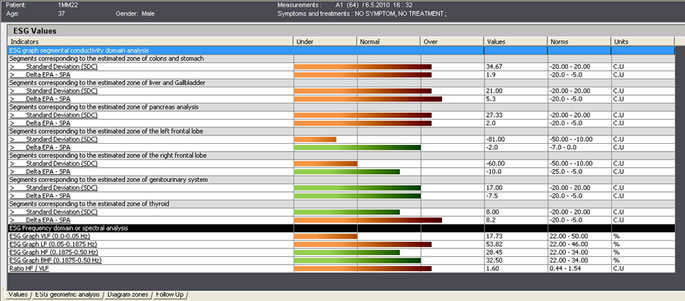
ES-Teck test performed on Male, 34 in May. All 9 measured areas are out of balance.
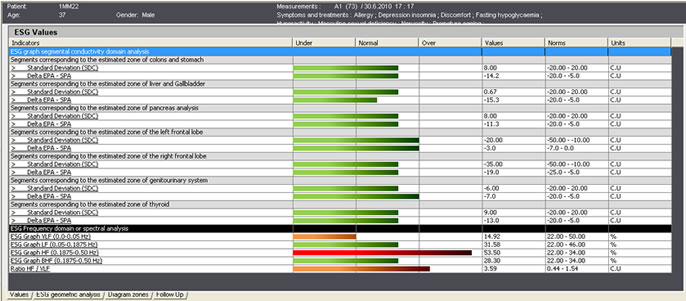
ES-Teck test performed on Male, 34 in June, after using PTD according to the test in May.
Supportive Test Methods
Whilst the EIS formed the main validation vehicle the following have also been used in various aspects of PTD validation for specific programs and to ascertain effects beyond direct cellular vitality.
PTD effectiveness with various FDA registered and CE Medical Class I & IIa devices.
Prognos; Meridian Diagnosis Device
NES: Health Assessment Device
Varicor: Heart Rate Variability
RD: Regulation Diagnostic Device (Fritz Albert Popp)
EM Wave Heart-math
GDV /EPC Kirlian Camera (Prof Konstatin Korotkov)
PIP Aura Camera
Infrared Thermograph Screening
Dark field microscope
